Robert Capodilupo & Jacob James Rich*
Introduction
The opioid crisis continues to ravage communities across the United States. In 2020, a record 91,799 people died of a drug overdose,[1] with 68,630 of these deaths attributed to opioids.[2] Even as policymakers appropriate unprecedented resources to reduce drug-related mortality,[3] the overdose crisis persists with no end in sight.
In her Yale Law & Policy Review Article, The Prescription Abuse Prevention Act: A New Federal Statute to Criminalize Overprescribing Opioids, Rebecca A. Delfino proposes novel legislation to reform how the federal government prosecutes doctors who overprescribe controlled substances.[4] According to Delfino, her Prescription Abuse Prevention Act (PAPA) would improve on current law by “offering a more specific and contextual legal framework focused on the expertise of the medical prescriber … [to] provide much-needed clarity in the law, thereby allowing legitimate prescribers to continue to treat patients in pain.”[5]
While PAPA has the noble intention of punishing doctors who knowingly overprescribe drugs that cause the death of a patient, its unpredictable implied-malice standard[6] will only further breed uncertainty for physicians.[7] In turn, as physicians indiscriminately reduce prescribing to avoid potential liability under PAPA, legitimate patients who can no longer access opioids legally may turn to the black market to meet their medical needs, increasing their risks of overdosing on more dangerous opioids like heroin and illicit fentanyl.[8] Because PAPA fails to clarify the criminal-liability standard for physicians, it will not ameliorate the opioid crisis and may very well exacerbate it by orienting both medical and recreational users to the illicit market.
I. Correcting the Record on the Opioid Crisis
Before turning to how PAPA could yield harmful unintended consequences, we would first like to address the errors in Delfino’s Article that mischaracterize the nature and causes of the opioid crisis. Because the Article’s policy prescription relies on the premise that the “all-too-common conduct [of] overprescribing opioids”[9] is a leading cause of overdose mortality,[10] it is necessary to evaluate the validity of this claim.
This premise is more tenuous than Delfino suggests. Beyond hairsplitting over the Article’s at-times inaccurate history of American drug policy,[11] we are concerned with its problematic mischaracterization of the rise of opioid prescribing in the United States and the state of the relevant literature discussing addiction—especially that which was available in the 1990s when OxyContin first entered the market. Like many popular accounts of the opioid crisis,[12] Delfino claims that the now-infamous Porter & Jick study[13] “laid the groundwork for the marketing practices that spawned the opioid crisis in the United States.”[14] While Porter & Jick’s letter to the editor in the New England Journal of Medicine itself presents insufficient evidence to support the efficacy of opioid treatments—and was incautiously misrepresented by pharmaceutical companies “to minimize the risk of addiction in the use of opioids”[15]—Delfino is wrong to suggest that there was a “dearth of peer-reviewed replicable scientific research and clinical studies on the efficacious applications of opioids.”[16] In fact, many studies published before the introduction of OxyContin in 1996 supported Porter & Jick’s findings that opioid addiction is rare in medical patients with no history of addiction.[17] More recent research has supported the specific claim that the rate of addiction for patients prescribed opioids with no history of substance abuse is less than one percent.[18] However, Delfino characterizes the literature in the 1980s and 1990s as a “research gap filled to a large extent by the Porter and Jick letter” and blames “[t]he diminished role of the federal government in funding drug science.”[19] Neither of these statements is true. In addition to the aforementioned literature indicating the efficacy of opioids during this period, funding for the National Institutes of Health—the government agency responsible for medical research—quadrupled between 1980 and 1999.[20]
As much of the literature from both the late-twentieth century and the present day share the letter’s conclusion that the “development of addiction is rare in medical patients with no history of addiction,”[21] it is inaccurate for Delfino to describe this letter as the preeminent source of the academy’s understanding of opioids without acknowledging the breadth of the relevant literature.[22] Though Porter & Jick’s study was not especially rigorous, Delfino’s neglect of considering more thoroughly researched findings from that period renders her account of the evidence used to justify early opioid prescribing incomplete.
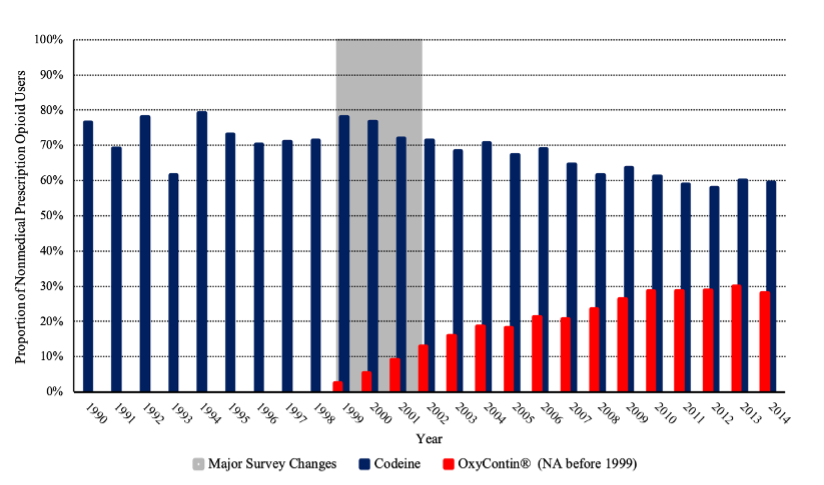
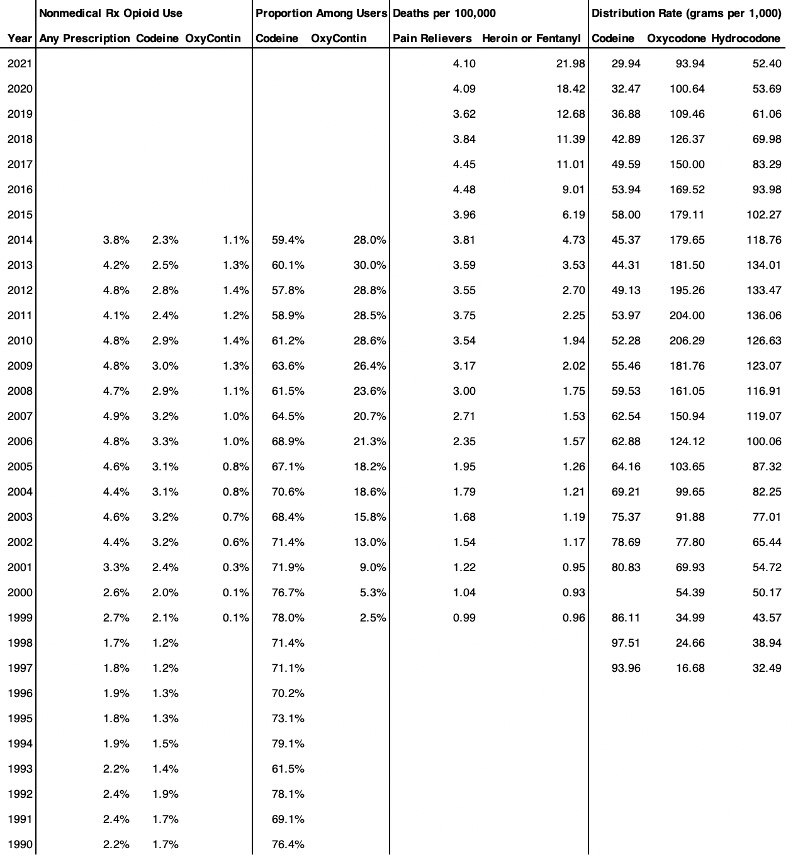
Indeed, Delfino’s focus on the relationship between physician prescribing and opioid misuse might lead one to believe that “nonmedical pain reliever use,” the type of opioid abuse that is related to overprescribing, has been increasing for some time. On the contrary, nonmedical prescription-pain-reliever use has remained overly stable for at least the past two decades.[23] Although OxyContin was introduced in 1996 and Purdue Pharma’s marketing campaign subsequently increased its market share within the industry of pain relievers that contain oxycodone,[24] only 9.0% of all nonmedical opioid users in 2001 reported ever using OxyContin during their lifetime.[25] A review by Kolodny et al. describes a rise in nonmedical opioid use following the introduction of OxyContin, with over 2.5 million people initiating first use of opioids in 2001.[26] However, much of this reported rise was likely due to major changes in survey methodology[27] and any real change would have been almost entirely driven by codeine, which 71.9% of past-year nonmedical prescription opioid users reported using during their lifetimes in 2001.[28] Indeed, this reported spike in nonmedical pain reliever use was accompanied by new developments in the entertainment industry said to “glamorize and promote the mixture” of codeine with soft drinks,[29] which motivated the Drug Enforcement Administration (DEA) to indict a group of medical professionals for illegally distributing 1.4 million tablets of hydrocodone and 2,100 gallons of promethazine with codeine in 2004.[30]
Overall, it is not clear that nonmedical opioid use has significantly changed since 1990. Even if the one reported spike in nonmedical pain reliever use between 1999 and 2001 was not entirely due to changes in survey design, this increase was not due to OxyContin exposure.
Figure 1—Percentage of the Population Consuming Prescription Opioid Pain Relievers in the Past Year for Nonmedical Purposes by Lifetime Use
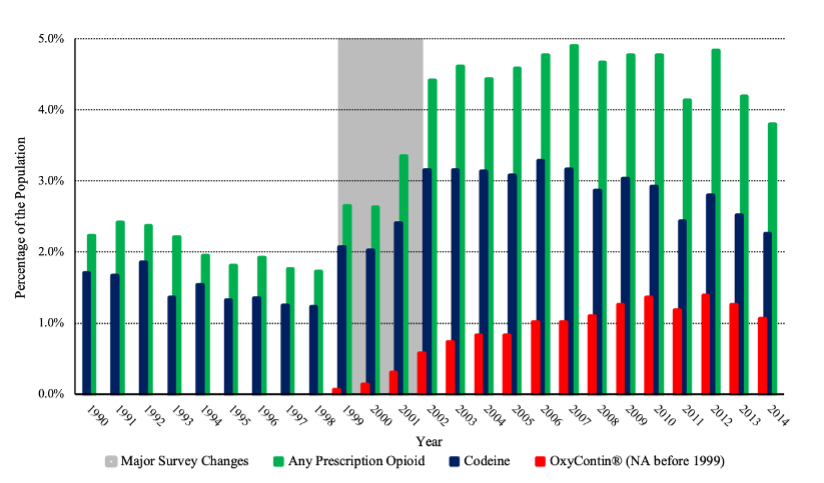
Figure 2—Proportion of Past-Year Nonmedical Prescription Opioid Users Who Have Used Codeine or OxyContin® During Their Lifetimes
But most damaging to Delfino’s normative project is its incorrect statement that “[s]eventy percent of [opioid overdose] deaths involve an opioid that a doctor legally prescribed.”[31] One can only arrive at this claim by treating synthetic opioids, like fentanyl, as “legally prescribed” drugs—a practice that is rejected by the relevant literature for inaccurately representing the source of most fentanyl.[32] According to a report published by the Centers for Disease Control & Prevention (CDC), “only a small percentage of fentanyl deaths had evidence consistent with prescription fentanyl,” while the vast majority of these deaths derive from “[i]llicitly manufactured fentanyl.”[33] By August 2017, the CDC had formally removed fentanyl from the definition of prescription opioid mortality.[34]
Moreover, to count all cases of fentanyl-overdose deaths as those caused by legally prescribed controlled substances inflates the fraction of deaths attributable to prescription opioids.[35] If policymakers want to use legislation to reduce overdose deaths, then it is critical for them to know where opioids that disproportionately lead to mortality are sourced. Prescription opioids have not accounted for a majority of opioid-overdose deaths since 2014 and have not represented a majority of total drug-related deaths since substance-specific data have been available.[36] Instead, illicit fentanyl is the driving force behind this most recent wave of the opioid crisis. In fact, the CDC wrote in JAMA that fentanyl alone accounted for “nearly all the increase in drug overdose deaths from 2015 to 2016.”[37] This has held for every year following.[38]
Figure 3—Opioid-Related Mortality Rate by Substance
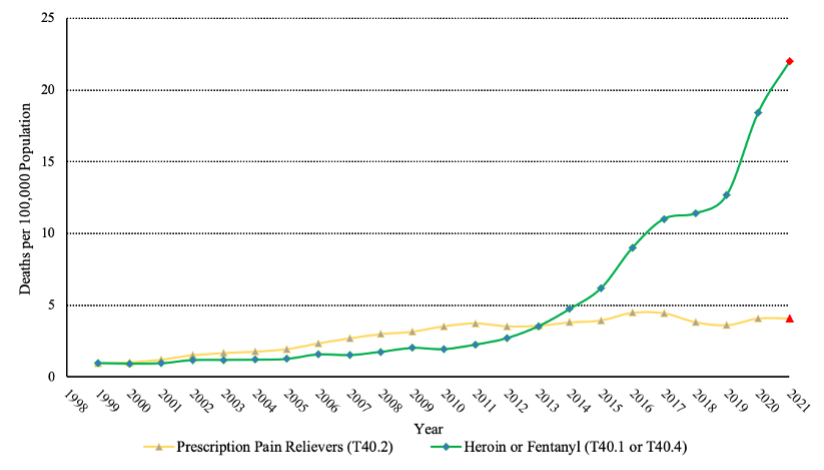
While opioid prescribing has declined over the past decade,[39] total opioid deaths have skyrocketed because of a spike in illicit-opioid overdoses. Thus, policies oriented at combatting prescription-opioid-overdose deaths may be ineffective at, or even counterproductive to, reducing the illicit-opioid overdoses that are primarily influencing the crisis. Although multiple studies find that certain types of prescription drug monitoring programs (PDMPs)—state-level databases that record the prescribing history between physicians and their patients for auditing purposes—are associated with reductions in opioid prescribing and prescription-opioid deaths, this literature suspects that their implementation is also associated with an increase in total drug-overdose deaths, driven by a larger increase in deaths caused by heroin and fentanyl.[40] Several other studies show that policies aimed at reducing opioid prescribing, in an effort to lower prescription-opioid-overdose deaths, at minimum lead to a rise in illicit-opioid-overdose deaths.[41] Though it may seem counterintuitive that policies effective in reducing the availability of prescription opioids can increase overdose deaths, opioid-related mortality has increased amid a decrease in opioid prescribing.
Figure 4—Opioid Prescribing by Substance
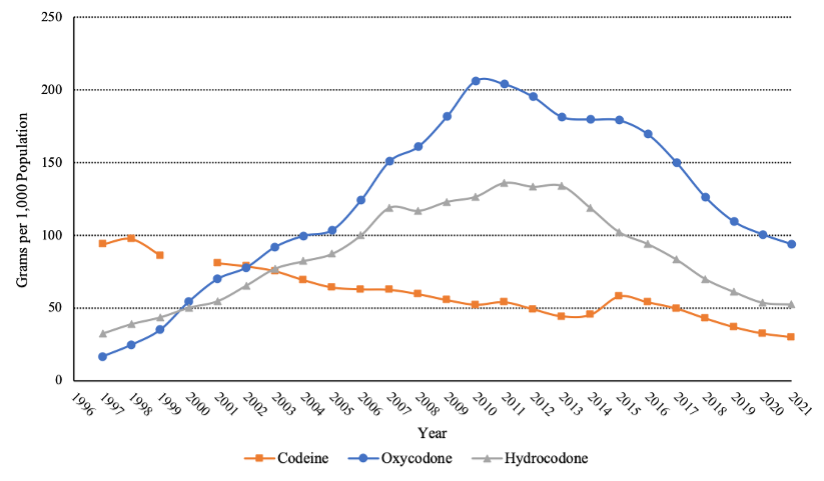
This outcome likely occurs because users cut off from legal channels of prescribed, quality-controlled drugs are often driven toward the black market to supplement their demand for opioids,[42] where drugs are far more dangerous. As one account explains, “[q]uality control is poor in underground markets because . . . [c]onsumers cannot easily assess the purity of the products they consume,” increasing the likelihood that they “accidentally take high-dose drugs or versions laced with more potent opioids like fentanyl.”[43] Because of these inherent dangers of the black market, policies that unduly restrict access to legal channels of prescribing appear to exacerbate overdoses from illicit opioids—as is reflected by the rapid rise in fentanyl deaths in the wake of these policy interventions.
Overall, Delfino’s failure to properly exclude fentanyl as a “legally prescribed”[44] opioid constructs a false premise that prescription opioids are the principal cause for the current wave of the opioid crisis. This mischaracterization undermines the cogency of her policy proposal and could potentially yield the devastating unintended consequence of increasing overdose deaths.
II. The Potential Unintended Consequences of the Prescription Abuse Prevention Act
Interventions that target the doctor-patient relationship bear the risk of yielding unintended consequences, especially when an addictive substance is being regulated.[45] The most harmful possible consequence of PAPA is that it would create a “chilling effect,” as physicians are deterred “from prescribing opioids to successfully treat a patient’s pain … due to the potentially negative influence of drug enforcement agents monitoring their prescribing behaviors.”[46] Delfino even acknowledges that the “lack of clarity” created by haphazard prosecution under the Controlled Substances Act (CSA) “causes healthcare providers to refrain from prescribing opioid drugs even for legitimate medical purposes.”[47] As such, PAPA is meant to represent “a clear statute that targets specific and defined conduct [and] give[s] physicians the freedom to alleviate legitimate chronic pain without fear of criminal ramifications.”[48]
Yet PAPA would only increase an appropriate prescriber’s chance of facing prosecution as compared to the current CSA regime. According to the proposed statute, “[a] health care professional who … acting with implied malice, proximately causes the death of another by knowingly and intentionally prescribing, distributing, bartering, delivering, exchanging, or administering a controlled substance classified in Controlled Substances Act … is guilty of murder in the second degree.”[49] Literally, PAPA, like the CSA,[50] offers an intent or knowledge mens rea standard. However, the proposed statute’s addition of an “implied malice” standard, and its capacious definition of it, could hold every prescriber who has a patient overdose liable.[51] By design, “the implied malice standard … embeds a doctor’s expert knowledge, practice experience, and specialized medical training evidence into the offense,” creating a “presumption of knowledge” for all prescribing physicians.[52]
Moreover, any overdose death following an opioid prescription could potentially qualify as a criminal offense under PAPA. Delfino appears confident that the additional need for the prosecution to prove “other evidence showing that the actor … deliberately acted with conscious disregard for human life”[53] would ameliorate “unjust outcomes afforded to some doctors” prosecuted under the CSA for legitimate prescribing activity.[54] This conclusion is far from certain.
According to Delfino, PAPA improves upon the CSA’s “legitimate medical treatment” standard by requiring jurors to instead “consider the subjective risk that the physician was able to assess in light of their knowledge and expertise from their medical training.”[55] As PAPA already presumes that a prescribing physician has sufficient knowledge, the only path towards acquittal is contingent on disproving whether “the facts available to the prescriber about the patient” establishes his conscious disregard for human life.[56] But the text of PAPA does not provide any guidance as to what “other evidence”[57] is sufficient for this purpose or how this inquiry is substantively different from that analyzing the legitimacy of the prescription. All PAPA does is shift the jury test from “an ad hoc analysis to determine whether physicians prescribed opioids for a legitimate medical purpose” to an equally amorphous ad hoc test of whether the prescriber “deliberately acted with conscious disregard for human life.”[58]
Lastly, it is unclear whether the presented “unjust” CSA cases would have turned out differently under PAPA. For instance, in United States v. Miller, a physician was convicted under the CSA after he allegedly “continued prescribing narcotics to patients despite the presence of clear red flags of drug abuse[,] … prescribed controlled substances when there were contraindications against use[,] … [and] ‘relentlessly continued’ prescribing controlled substances to a patient who had been admitted to the hospital with an overdose.”[59] Because PAPA aims to do away with the CSA’s “undefined ‘legitimate medical treatment’ standard,” Delfino suggests that Miller would have resulted in acquittal, as “the evidence of criminality was weak.”[60] However, given Dr. Miller’s medical training and presumed knowledge of the dangers of opioids under PAPA, it is not obvious that a jury would not consider his aforementioned prescribing practices as sufficient additional evidence to establish implied malice, and thus, liability.
In one respect, however, PAPA may improve on the current CSA regime. As Delfino notes, “PAPA would also impose criminal liability on pill mill doctors who escaped liability under the CSA and similar state statutes” by focusing on proximate, rather than but-for, causation.[61] In theory, a legal regime that is better suited for prosecuting the most egregious examples of non-medical overprescribing is desirable. Still, “[m]ost … prescription opioids … are not coming from ‘pill mills.’”[62] As such, undermining the significance of this benefit, especially considering the costs brought on by PAPA’s likely chilling effect. Furthermore, though closing down pill mills and punishing the doctors running them may seem advantageous, evidence suggests that heroin and fentanyl deaths may actually increase as pill mills are closed and their former customers turn to the black market.[63]
Instead of clarifying the law to increase opioid access to legitimate pain patients, as Delfino hopes to accomplish, PAPA appears to preserve a physician’s current incentive not to prescribe opioids in any circumstance. The law’s scant explanation of the implied-malice standard and presumption of knowledge perpetuate uncertainty for prescribing physicians—and perhaps even increase a physician’s liability for legitimate treatments—by replacing one amorphous standard with another.
If physicians face the potential of criminal liability for prescribing legitimate treatments, many physicians will choose to cease prescribing altogether[64] and patients will suffer.[65] According to one addictologist, “patients [who are cut off from legal-prescribing channels] are going to have a physical dependency, and some of them might be able to wean themselves off, but some are going to buy heroin on the street and some of that is going to be laced with fentanyl.”[66] This may explain why much of the increase in overdose deaths is among patients with disabilities,[67] as those in most need of pain medication for treatment switch to heroin when prescription opioids become less accessible.[68]
Thus, while PAPA’s intention of clarifying criminal liability for prescribing physicians is noble, its failure to do so—demonstrated by its muddled implied-malice standard and burdensome presumption of physician knowledge—will likely perpetuate a chilling effect on prescribing and further orient patients towards the dangerous illicit opioids that are driving the crisis.
Conclusion: A Better Path Towards Harm Reduction
Speaking to the political salience of her proposal, Delfino states that “in terms of optics and symbolism, a federal criminal statute focused expressly and exclusively on opioid prescribers signals to the public … that the opioid crisis is of such monumental significance that it deserves unique treatment.”[69] In times of crisis, it is common for governments to pursue policy interventions,[70] but the long-term effects are often undesirable.[71]
The adoption of PAPA’s implied-malice standard would only further complicate the physician-patient relationship by increasing legal uncertainty and disincentivizing doctors from providing opioid treatments to addiction and pain patients alike. In turn, those users who are cut off from legal channels of prescribing would need to turn to the black market to meet their demand for opioids, increasing their risk of fatal overdose.
Our criticisms of PAPA, however, should not be taken as an endorsement of the current regime under the CSA. We agree with Delfino that the “[p]rosecution of doctors under the federal CSA has been problematic in several respects.”[72] However, this is because the CSA has made it more difficult for doctors to provide legitimate opioid treatments for patients, out of fear of prosecution. As such, instead of abandoning the CSA regime, we recommend reforming it—specifically through adopting a more capacious definition of what constitutes a “legitimate medical purpose.”[73] This appears to be the path taken by the Supreme Court in the recent case, Xiulu Ruan v. United States. There, the Court interpreted the CSA’s prohibition on doctors “knowingly” dispensing opioids outside of the “usual course of his professional practice” to mean that, in order to face liability, doctors must know the prescription was outside of the usual course of practice, rather than simply know he was prescribing the substance.[74] This interpretation may work towards reducing the chilling effect discouraging opioid treatments, as physicians can only face liability under the CSA if the government can “prove beyond a reasonable doubt that the defendant knowingly or intentionally acted in an unauthorized manner.”[75]
Still, long-term maintenance of patients on opioids—especially illicit opioids like heroin—is still considered to be outside “the usual course of professional practice,” and thus remains an unauthorized procedure prohibited by the CSA.[76] Maintaining those suffering from addiction on opioid regimes is considered a legitimate practice in many other countries.[77] For example, qualifying physicians in Canada are allowed to prescribe long-term regimens—and even pharmaceutical-grade heroin—to “people who are severely addicted to opioids.”[78] The logic behind these programs follows from the idea that the harms of drug use will be reduced if a user can acquire the substance legally.[79] Recognizing that many people who are highly addicted to opioids are going to seek out these drugs regardless of legality,[80] these programs seek to reduce the harms of drug use by ensuring that users are given an unadulterated substance.
In the United States, legalizing heroin-assisted treatment would require both a rescheduling of heroin from Schedule 1 to Schedule 2, and an agency reinterpretation of the CSA to recognize this practice as legitimate.[81] In turn, reforming the CSA to allow for heroin-assisted treatment would likely have a significant effect on reducing overdose deaths in the United States. As one review of the outcomes of heroin-assisted treatment in Europe concludes, these programs are associated with “substantial improvement in health and well-being of the patients … [including] a major reduction in the extent of continued injecting of ‘street’ heroin, improvements in general health, psychological well-being and social functioning, as well as major disengagement from criminal activities.”[82] And, crucially, heroin-assisted treatment is associated with “reductions in illicit heroin use,” suggesting that users are at a decreased risk for overdoses caused by laced substances.[83]
The current nature of the opioid crisis demands solutions that recognize the nuances of the situation. PAPA would only perpetuate the current failed approach by incentivizing the use of black-market opioids, the principal driver of overdose deaths. Instead of limiting access to legal opioids, public policy should be working towards expanding the extent to which opioid users can continue to be treated by physicians in a safer, supervised manner. Amending the CSA to allow for the expansion of legal, legitimate maintenance treatment, rather than increasing the scrutiny of prescribing in an imprecise and ambiguous manner, poses the best policy framework for reducing the harms of opioid use.
Table 1—Data for Illustrations
* Robert Capodilupo, Yale Law School, J.D. expected Spring 2023; M.Phil., Magdalene College, University of Cambridge, A.B. magna cum laude in Government, Harvard College. Jacob James Rich, Cleveland Clinic Center for Value-Based Care Research; Reason Foundation Drug Policy Project; Case Western Reserve University School of Medicine, Ph.D. expected Spring 2025; M.A., Eastern Michigan University; B.S., Eastern Michigan University. The Authors would like to thank Professor Jonathan H. Adler, Professor Jeffrey A. Miron, Dr. Jeffrey A. Singer, and Professor Kate Stith for their helpful feedback on earlier drafts of this Essay. The Authors are particularly grateful to Isaiah Ogren for his fantastic work on this Essay as Executive Editor for Inter Alia and to Karissa Kang and Amir Perk for demonstrating exceptional academic integrity in agreeing to publish this response.
[1] Holly Hedegaard, Arialdi M. Miniño, Merianne Rose Spencer & Margaret Warner, Drug Overdose Deaths in the United States, 1999-2020, at 1 (NCHS Data Brief No. 428, 2021).
[2] Overdose Death Rates, Nat. Inst. on Drug Abuse, https://nida.nih.gov/drug-topics/trends-statistics/overdose-death-rates, [https://perma.cc/C3FG-83EE].
[3] See, e.g., STOP Fentanyl Act, H.R. 2366, 117th Cong. (2021); Using Data to Prevent Opioid Diversion Act, S.2823, 115th Cong. (2018); SUPPORT Act, Pub. L. No. 115-271, 132 Stat. 3894 (2018); see also Agata Dabrowska, Victoria R. Green & Lisa A. Sacco, Cong. Rsch. Serv., R45405, The SUPPORT for Patients and Communities Act (P.L. 115-271): Food and Drug Administration and Controlled Substance Provisions 1 (2018) (“Broadly, the legislation imposes tighter oversight of opioid production and distribution; imposes additional reporting and safeguards to address fraud; and limits coverage of prescription opioids, while expanding coverage of and access to opioid addiction treatment services.”).
[4] Rebecca A. Delfino, The Prescription Abuse Prevention Act: A New Federal Statute to Criminalize Overprescribing Opioids, 39 Yale L. & Pol’y Rev. 347 (2021).
[5] Id. at 406-08.
[6] See id. at 407-11.
[7] See, e.g., Charles Schmidt, Experts Worry About Chilling Effect of Federal Regulations on Treating Pain, 97 J. Nat’l Cancer Inst. 554, 554-55 (2005).
[8] See Jeffrey Miron, Greg Sollenberger & Laura Nicolae, Overdosing on Regulation: How Government Caused the Opioid Epidemic 3-4 (Cato Inst., Pol’y Analysis No. 864, 2019).
[9] Delfino, supra note 4, at 352.
[10] See id. at 350 (“There is a growing sense that those responsible for instigating the opioid crisis [are] drug companies and doctors who prescribe these drugs”).
[11] Many of Defino’s assertions regarding the evolution of drug regulation in the United States are either inaccurate or lacking nuance. For example, the Article states that the Opium Exclusion Act of 1909 “was the first government regulation of opioids.” Delfino, supra note 4, at 354. While this law was the first federal prohibition on opiate use, Congress had previously regulated opiate availability through taxation and tariffs, see Audrey Redford & Benjamin Powell, Dynamics of Intervention in the War on Drugs: The Buildup to the Harrison Act of 1914, 20 Indep. Rev. 509, 512-17 (2016), and state and local governments had already begun to outlaw opium by the end of the nineteenth century. See Gregory Yee Mark, Racial, Economic and Political Factors in the Development of America’s First Drug Laws, 10 Issues Criminology 49, 61-63 (1975). Delfino also wrongly states that “in 1938, Congress created the Food and Drug Administration (FDA) to monitor and regulate drugs and their safety before sale in the United States.” Delfino, supra note 4, at 355. While the FDA was granted additional regulatory power over food, cosmetics, and drugs in 1938, see Federal Food, Drug, and Cosmetic Act, Pub. L. No. 75-717, 52 Stat. 1040 (1938), the agency existed as the United States Department of Agriculture Bureau of Chemistry since 1862 and took on its modern incarnation in 1906 with the adoption of the Pure Food and Drug Act. Pure Food and Drug Act, Pub. L. No. 59-384, 34 Stat. 768 (1906); Milestones in U.S. Food and Drug Law, U.S. Food & Drug Admin. (Jan. 31, 2018), https://www.fda.gov/about-fda/fda-history/milestones-us-food-and-drug-law, [https://perma.cc/G3WY-UTVM].
[12] See, e.g., Sarah Zhang, The One-Paragraph Letter From 1980 That Fueled the Opioid Crisis, The Atlantic (June 2, 2017), https://www.theatlantic.com/health/archive/2017/06/nejm-letter-opioids/5…, [https://perma.cc/7582-ECBH] .
[13] See Jane Porter & Hershel Jick, Addiction Rare in Patients Treated with Narcotics, 302 N. Engl. J. Med. 123, 123 (1980).
[14] Delfino, supra note 4, at 359.
[15] Art Van Zee, The Promotion and Marketing of OxyContin: Commercial Triumph, Public Health Tragedy, 99 Am. J. Pub. Health. 221, 223 (2009).
[16] Delfino, supra note 4, at 417.
[17] See, e.g., Russell K. Portenoy & Kathleen M. Foley, Chronic Use of Opioid Analgesics in Non-malignant Pain: Report of 38 Cases, 25 Pain 171, 178-82 (1986); Randal D. France, Bruno J. Urban & Frances J. Keefe, Long-term Use of Narcotic Analgesics in Chronic Pain, 19 Soc. Sci. Med. 1379, 1381 (1984); Russell K. Portenoy & Nessa Coyle, Controversies in the Long-Term Management of Analgesic Therapy in Patients with Advanced Cancer, 5 J. Pain & Symptom Mgmt. 307, 315-17 (1990); Michael Zenz, Michael Strumpf & Michael Tryba, Long-term Oral Opioid Therapy in Patients with Chronic Nonmalignant Pain, 7 J. Pain & Symptom Mgmt. 69, 75-76 (1992); Ronald M. Kanner & Kathleen M. Foley, Patterns of Narcotic Drug Use in a Cancer Pain Clinic, 362 Annals N.Y. Acad. Sci. 161, 166 (1981).
[18] See, e.g., David A. Fishbain, Brandly Cole, John Lewis, Hubert L. Rosomoff & R. Steele Rosomoff, What Percentage of Chronic Nonmalignant Pain Patients Exposed to Chronic Opioid Analgesic Therapy Develop Abuse/Addiction and/or Aberrant Drug-related Behaviors? A Structured Evidence-based Review, 9 Pain Med. 444, 452-55 (2008); Meredith Noble et al., Long-term opioid management for chronic noncancer pain, at 8 (Cochrane Database of Systematic Revs., 2010); Gabriel A. Brat et al., Postsurgical Prescriptions for Opioid Naive Patients and Association with Overdose and Misuse: Retrospective Cohort Study, 360 Brit. Med. J. j5790, at 5-8 (2018). Other studies have found a higher addiction rate, “depending on the criteria used and the subpopulation studied.” Van Zee, supra note 15, at 233. See, e.g., Kyriaki Kouyanou, Charles E. Pither & Simon Wesseley, Medication Misuse, Abuse and Dependence in Chronic Pain Patients, 45 J. Psychomatic Rsch. 497, 501-03 (1997); David T. Cowan, Jennifer Wilson-Barnett, Peter Griffiths & Laurie G. Allan, A Survey of Chronic Noncancer Pain Patients Prescribed Opioid Analgesics, 4 Pain Med. 340, 347-48 (2003); Nathaniel Katz, Opioids: After Thousands of Years, Still Getting to Know You, 23 Clinical J. Pain 303, 304 (2007).
[19] Delfino, supra note 4, at 369.
[20] See Appropriations History by Institute/Center (1938 to Present), Nat’l Insts. of Health, https://officeofbudget.od.nih.gov/approp_hist.html, [https://perma.cc/V9D6-UWGH]; Kavya Sekar, Cong. Rsch. Serv., R43341, National Institutes of Health (NIH) Funding: FY1996-FY2023, at 11-12 (2022).
[21] Porter & Jick, supra note 13, at 123.
[22] Delfino, supra note 4, at 369.
[23] See infra Figures 1 and 2. Data compiled from National Survey on Drug Use and Health, SAMHSA, https://www.datafiles.samhsa.gov/dataset/national-survey-drug-use-and-he…, [https://perma.cc/L9R6-FEHX] [hereinafter SAMHSA, NSDUH Data] and the National Household Survey on Drug Abuse, SAMHSA, https://www.datafiles.samhsa.gov/dataset/national-household-survey-drug-…, [https://perma.cc/E7UF-HTNE] [hereinafter SAMHSA, NHSDA Data]. OxyContin (“oxycont2” or “iroxyrc”) was not recorded in these data until 1999. The gray shaded region represents major changes in survey methodology. The complex survey design accounts for weights (“analwt” or “analwt_c”), strata (“vestr”), and primary sampling units (“verep”). Codeine use includes nonmedical users of tylenol with codeine (“tylcod” or “darvtyl2”) or codeine alone (“codeine” or “codeine2”). Nonmedical prescription opioid use in the past year (“anlyr” or “iranlrc”) is estimated among adults ages 18 and above (“irage”, “irage2”, or “age2”). For years 2015 to 2020, see SAMHSA, Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health, at 18 (HHS Pub. No. PEP21-07-01-003, NSDUH Series H-56, 2021).
[24] See Shiyu Zhang & Daniel Guth, The OxyContin Reformulation Revisited: New Evidence from Improved Definitions of Markets and Substitutes, at 8 (Jan. 2021) (unpublished manuscript), available at https://arxiv.org/abs/2101.01128, [https://perma.cc/M5HT-ZCXG].
[25] See SAMHSA, NSDUH Data, supra note 23; SAMHSA, NHSDA Data, supra note 23.
[26] See Andrew Kolodny et al., The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction, 36 Annual Rev. Pub. Health. 559, 562-63 (2015).
[27] Between 1999 and 2001, the National Survey on Drug Use and Health (NHSDA) was transitioning from written to computer-assisted data collection methods. SAMHSA warns against comparing incidence estimates (i.e. estimates of new drug users) to prior years during this period, claiming “These data should not be compared with previously published NHSDA data based on paper-and-pencil interviewing methods. Not only is the mode of data collection different for the incidence estimates produced prior to the 1999 NHSDA, but the estimation methodology has been revised as well.” See SAMHSA, Results from the 2001 National Household Survey on Drug Abuse. Volume I: Summary of National Findings [and] Volume II: Technical Appendices and Selected Data Tables [and] Volume III: Detailed Tables, at 43 (HHS Pub. No. SMA-02-3758; SMA-02-3759, 2002),
https://files.eric.ed.gov/fulltext/ED470404.pdf, [https://perma.cc/ZM75-NM6T]. Estimates of new drug users between 1999 and 2001 might show inaccurate jumps, see infra Figure 1. However, the proportion of drug use due to various substances among the drug users is relatively accurate, see infra Figure 2.
[28] See SAMHSA, NSDUH Data, supra note 23; SAMHSA, NHSDA Data, supra note 23.
[29] Press Release, U.S. Dep’t of Justice, Drug Alert Watch: Resurgence in Abuse of ‘Purple Drank,’ at 2 (Feb. 15, 2011), https://www.justice.gov/archive/ndic/pubs43/43924/sw0008p.pdf, [https://perma.cc/L7B9-EFDC]; see also William N. Elwood, Sticky Business: Patterns of Procurement and Misuse of Prescription Cough Syrup in Houston, 33 J. Psychoactive Drugs. 121, 123-130 (2001) (describing the prevalence of codeine use around this time).
[30] Press Release, DEA, Houston Doctor and 6 Local Pharmacists Charged in Conspiracy to Illegally Distribute Narcotics (Oct. 1, 2004), https://www.dea.gov/sites/default/files/pubs/states/newsrel/houston10010…, [https://perma.cc/9H6K-WNGE].
[31] Delfino, supra note 4, at 347.
[32] See, e.g., Julie K. O’Donnell, John Halpin, Christine L. Mattson, Bruce A. Goldberger & R. Matthew Gladden, Deaths Involving Fentanyl, Fentanyl Analogs, and U-47700—10 States, July-December 2016, 66 Morbidity & Mortality Wkly. Rpt. 1197, 1201 (2017) (noting “[i]llicitly manufactured fentanyl … is primarily responsible for this rapid increase” in overdose deaths); Puja Seth, Rose A. Rudd, Rita K. Noonan, Tamara M. Haegerich, Quantifying the Epidemic of Prescription Opioid Overdose Deaths, 108 Am. J. Pub. Health 500, 500-02 (2018) (“[R]ates of prescription opioid–involved deaths estimated with the traditional method may have been inflated in recent years because of the increase in death rates involving synthetic opioids (e.g., fentanyl).”).
[33] O’Donnell et al., supra note 32, at 1201.
[34] See Annual Surveillance Report of Drug Related Risks and Outcomes, Ctrs. Disease Control & Prevention, at 35 (2017), https://www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-rep…, [https://perma.cc/W89C-KFE7] (omitting fentanyl as a prescription opioid).
[35] While Delfino claims that legally prescribed opioids accounted for seventy percent of “opioid overdose” deaths “now” and “nearly two-thirds” of all “drug overdose[]” deaths in 2018, see Delfino, supra note 4, at 347-49, those figure are overstated. When properly classifying fentanyl as an illicit drug, prescription opioids were only responsible for about twenty-four percent of opioid-overdose deaths and eighteen percent of all drug-overdose deaths in 2020. In 2018, prescription opioids were associated with thirty-two percent of opioid deaths and twenty-two percent of all drug deaths. See Ctrs. Disease Control & Prevention, Multiple Cause of Death, 1999-2020 Request, https://wonder.cdc.gov/controller/datarequest/D77, [https://perma.cc/N5TR-3BU3] [hereinafter Ctrs. Disease Control & Prevention, Multiple Cause of Death] (Drug-overdose deaths correspond to Underlying Cause of Deaths Codes X40–X44, X60–X64, X85, and Y10–Y14, and Multiple Cause of Death Codes T40.0 (opium), T40.1 (heroin), T40.2 (natural opioid analgesics), T40.3 (methadone), T40.4 (other synthetic narcotics) T40.5 (cocaine), and T40.6 (other and unspecified narcotics).). These data are reported in Figure 3, infra. Year 2021 mortality data are preliminary estimates, see Ctrs. Disease Control & Prevention, Provisional Mortality Statistics, 2018 through Last Month Results, Request, https://wonder.cdc.gov/controller/datarequest/D77, [https://perma.cc/EV8P-4S3G].
[36] See Ctrs. Disease Control & Prevention, Multiple Cause of Death, supra note 35. In 1999, the CDC switched from ICD-9 to ICD-10 codes for classifying causes of death. See Ctrs. Disease Control & Prevention, International Classification of Diseases, (ICD-10-CM/PCS) Transition –Background, https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm, [https://perma.cc/AN3Y-W83A]. Thus, data from before 1999 are not directly comparable to those from after. For the prescription opioids definition, natural opioid analgesic “pain relievers” (T40.2) and methadone (T40.3) are combined.
[37] Deborah Dowell, Rita K. Noonan & Debra Houry, Underlying Factors in Drug Overdose Deaths, 318 J. Am. Med. Ass’n 2295, 2295 (2017).
[38] See Jacob James Rich & Robert Capodilupo, Opioid Prescribing Mediating State Policy Intervention Effects on Drug Overdose Mortality, at 4 (unpublished manuscript) https://doi.org/10.1101/2021.05.13.21257109, [https://perma.cc/5VNJ-SJY5] [hereinafter Rich & Capodilupo, PDMP Letter]; see also Jacob James Rich & Robert Capodilupo, Prescription Drug Monitoring Programs: PDMP Effects on Opioid Prescribing and Drug Overdose Mortality 18-19 (Reason Found., Pol’y Stud., 2021), https://reason.org/wp-content/uploads/prescription-drug-monitoring-progr…, [https://perma.cc/PLS3-G82C] [hereinafter Rich & Capodilupo, Policy Study] (“Though reductions in prescribing seem to contribute to increases in black market deaths, we do find strong evidence that they are associated with reductions in prescription opioid deaths… . [B]ut these small reductions are dwarfed by the massive increase in illicit opioid deaths.”).
[39] See infra Figure 4. These data were compiled from ARCOS Retail Drug Summary Reports, DEA, https://www.deadiversion.usdoj.gov/arcos/retail_drug_summary, [https://perma.cc/76UF-L74V].
[40] ARCOS Retail Drug Summary Reports, supra note 39; see also Byungkyu Lee et al., Systematic Evaluation of State Policy Interventions Targeting the US Opioid Epidemic, 2007-2018, 4 Jama Network Open e2036687, at 7-9 (2021) (providing statistical evidence for the claim that illicit-opioid overdose deaths increase in the wake of “interventions to control opioid prescriptions”); Rich & Capodilupo, PDMP Letter, supra note 38, at 2 (“Despite a 57.4% decrease in opioid prescriptionsfrom pharmacies since a peak in 2012, the opioid death rate has increased 105.8% through 2019, as the share of those deaths involving fentanyl increased from 16.4% to 72.9%.”).
[41] See, e.g., Richard Brown et al., Impact of New York Prescription Drug Monitoring Program, I-STOP, on Statewide Overdose Morbidity, 178 Drug & Alcohol Dependence 348, 350-51 (2017) (hypothesizing that increases in heroin deaths, despite reductions in opioid prescribing may be because “people resort to using heroin when they have a difficult time obtaining prescription opioids to satisfy their addiction”); Young Hee Nam, Dennis G. Shea, Yunfeng Shi, John R. Moran, State Prescription Drug Monitoring Programs and Fatal Drug Overdoses, 23 Am. J. Managed Care 297, 301 (2017) (“[O]ur results imply that PDMPs might be related to increases in drug overdose mortality rates attributable to illicit drugs … .”); Guohua Li et al., Prescription Drug Monitoring and Drug Overdose Mortality, 1 Injury Epidemiology 1, 7 (2014) (“[A]s prescription drugs become more difficult to procure, illicit drugs such as heroin may be substituted.”); Chris Delcher et al., Prescription and Illicit Opioid Deaths and the Prescription Drug Monitoring Program in Florida, 106 Am. J. Pub. Health e10, e10 (2016).
[42] Rich & Capodilupo, Policy Study, supra note 38, at 9.
[43] Miron, Sollenberger & Nicolae, supra note 8, at 3-4; see also Jeffrey A. Miron & Jeffrey Zwiebel, The Economic Case Against Drug Prohibition, 9 J. Econ. Persps. 175, 179 (1995) (“[A]ccidental poisonings and overdoses will occur more frequently in a prohibited market.”).
[44] Delfino, supra note 4, at 347.
[45] See Miron, Sollenberger & Nicolae, supra note 8, at 4.
[46] Richard M. Reisman et al., Prescription Opioid Usage and Abuse Relationships: An Evaluation of State Prescription Drug Monitoring Program Efficacy, 3 Substance Abuse: Rsch. & Treatment 41, 42 (2009); see also Dennis C. Turk, Michael C. Brody & Okifjui E. Akiko, Physicians’ Attitudes and Practices Regarding the Long-term Prescribing of Opioids for Non-Cancer Pain, 59 Pain 201, 206 (1996) (“[P]rescriptions of schedule II controlled substances for outpatients in a teaching hospital in Texas was reported to decrease by 60% after the state adopted the multiple prescription law in 1982… . Similarly reductions in prescribing of controlled substances declined by 57% in Rhode Island, 54% in New York, and 50% in Idaho after imposition of triplicate prescription programs.” (internal citations omitted)); Dennis Ross-Degnan et al., A Controlled Study of the Effects of State Surveillance on Indicators of Problematic and Non-Problematic Benzodiazepine Use in a Medicaid Population, 34 Int’l J. Psych. Med. 103, 117-19 (2004) (noting that increased state surveillance of prescribing of benzodiazepines in New York led to a decrease in treatment access for “twice as many non-problematic than problematic users”).
[47] Delfino, supra note 4, at 386; see also id. at 400-01 (“Chronic pain has notoriously been undertreated because … in light of the CSA and related laws, the fear of legal repercussions for overprescribing.”).
[48] Id. at 402.
[49] Id. at 407.
[50] See 21 U.S.C. § 841(a).
[51] PAPA states that “‘[i]mplied malice’ … may be shown by the possession of specialized medical or expert knowledge of the dangerousness and the addictive nature of the controlled substances … in addition to other evidence showing that the actor, knowing the act was dangerous to human life, deliberately acted with conscious disregard for human life.” Delfino, supra note 4, at 407.
[52] Id. at 409-10.
[53] Id. at 407.
[54] Id. at 410.
[55] Id. at 409.
[56] Id. at 410.
[57] Id. at 407.
[58] Id. at 391, 407.
[59] United States v. Miller, 891 F.3d 1220, 1227 (10th Cir. 2018) (internal citations omitted).
[60] Delfino, supra note 4, at 390, 410.
[61] Id. at 411.
[62] Darius A. Rastegar, “Prescription Monitoring Program and ‘Pill Mill’ Laws Have Had Modest Effects on Opioid Overprescribing in Florida,” B.U. AOD Health (Nov. 1, 2015), https://www.bu.edu/aodhealth/2015/11/01/prescription-monitoring-program-…, [https://perma.cc/BRP2-W8CV]; see also Mathew V. Kiang, Keith Humphreys, Mark R. Cullen & Sanjay Basu, Opioid Prescribing Patterns among Medical Providers in the United States, 2003-17: Retrospective, Observational Study, 368 BMJ 16968, at 1 (2020) (“In 2017, the top 1% of providers accounted for 49% of all opioid doses and 27% of all opioid prescriptions.”).
[63] See Delcher et al., supra note 40, at e10.
[64] See, e.g., Jayne O’Donnell & Ken Alltucker, Pain Patients Left in Anguish by Doctors ‘Terrified’ of Opioid Addiction, Despite CDC Change, USA Today (June 30, 2019, 4:55 PM), https://www.usatoday.com/story/news/health/2019/06/24/pain-patients-left…, [https://perma.cc/KKM5-R8RH] (“My pain management doctor said they cannot give me the medication because they could lose their license.”); US: Fears of Prescribing Hurt Chronic Pain Patients, Hum. Rgts. Watch (Dec. 18, 2018, 12:01 AM), https://www.hrw.org/news/2018/12/18/us-fears-prescribing-hurt-chronic-pa…, [https://perma.cc/R3K7-8FVW] (“Medical professionals interviewed by Human Rights Watch said they faced intense pressure to reduce opioid prescribing, even in cases where they believed a patient was benefitting from and taking their medication appropriately.”).
[65] See Red Lawhern, The CDC Opioid Guidelines Violate Standards of Science Research, Am. Council on Sci. & Health (Mar. 25, 2017), https://www.acsh.org/news/2017/03/25/cdc-opioid-guidelines-violate-stand… (predicting that due to increased scrutiny of opioid prescribing “more physicians will leave pain management practice, throwing thousands of patients into the street without medical referral or support when they go into opioid withdrawal”); Jill Heidi Osborne, The Chilling Effect of the CDC Guidelines for Chronic Pain 5-10 (2017), http://www.ic-network.com/1217/1217opt.pdf, [https://perma.cc/QU9W-X5HV].
[66] Brett Kelman, As Tennessee Pain Clinics Close, Some Desperate Patients May Switch to Heroin, Experts Say, Nashville Tennessean (July 6, 2018, 7:06 AM), https://www.tennessean.com/story/news/2018/07/05/comprehensive-pain-clin…, [https://perma.cc/AL4D-63UJ].
[67] See Yong-Fang Kuo, Mulaila A. Raji & James S. Goodwin, Association of Disability with Mortality from Opioid Overdose amoung US Medicare Adults, 2 JAMA Network Open e1915638, at 9 (2019) (finding “increased opioid overdose deaths among adults who qualified for Medicare because of disability”).
[68] See Zirui Song, Mortality Quadrupled Among Opioid-Driven Hospitalizations, Notably Within Lower-Income and Disabled White Populations, 36 Health Affs. 2054, 2059 (2017).
[69] Delfino, supra note 4, at 413.
[70] See Redford & Powell, supra note 11, at 511; Robert Higgs, Crisis and Leviathan: Critical Episodes in the Growth of American Government 17-19 (1987).
[71] Calvin B. Hooper, The Economy, Liberty, and the State 326-27 (1959).
[72] Delfino, supra note 4, at 386.
[73] 21 C.F.R. § 1306.04(a) (2022).
[74] Xiulu Ruan v. United States, 142 S. Ct. 2370, 2375-76 (2022) (first quoting 21 U.S.C. § 841(a), then quoting 21 C.F.R. § 1306.04(a) (2021)).
[75] Id. at 2382.
[76] United States v. Moore, 423 U.S. 122, 138 (1975) (discussing the relevant standard for criminal liability); see also Kelly K. Dineen & James M. DuBois, Between a Rock and a Hard Place: Can Physician Prescribe Opioids to Treat Pain Adequately While Avoiding Legal Sanction?, 42 Am. J. Law & Med. 7, 29-30 (2016) (detailing the CSA regime).
[77] See, e.g., Steve Rolles, Heroin-assisted Treatment in Switzerland: Successfully Regulating the Supply and Use of a High-risk Injectable Drug 1 (May 2016), http://fileserver.idpc.net/library/Heroin-assisted%20treatment%20Switzer…, [https://perma.cc/3992-4YGD] (“A number of countries – including Switzerland, the UK, Germany, the Netherlands, Australia and Canada – prescribe heroin for use under medical supervision, as part of successful [programs] to treat long-term users of illicit opioids.”).
[78] The Canadian Press, Canada Now Allows Prescription Heroin in Severe Opioid Addiction, CBC (Sept. 8, 2016, 11:01 AM), https://www.cbc.ca/news/canada/british-columbia/canada-now-allows-prescr…, [https://perma.cc/JZV2-M7EP]; see also Lucy Strang & Jirka Taylor, Heroin-Assisted Treatment and Supervised Drug Consumption Sites 5 (Rand Corp., Working Paper No. 1262, Dec. 2018), https://www.rand.org/content/dam/rand/pubs/working_papers/WR1200/WR1262/…, [https://perma.cc/5RET-2R8J] (providing an overview of heroin-assisted treatment in Canada).
[79] See Miron, Sollenberger & Nicolae, supra note 8, at 3.
[80] See John Strang, Teodora Groshkova & Nicola Metrebian, New Heroin-Assisted Treatment: Recent Evidence and Current Practices of Supervised Injectable Heroin Treatment in Europe and Beyond 25 (Euro. Monitoring Ctr. for Drugs & Drug Addiction, 2012), https://www.emcdda.europa.eu/system/files/publications/690/Heroin_Insigh…, [https://perma.cc/CV4A-4N8Y] (noting that heroin maintenance is only available for “long-term opiate users”); Beau Kilmer et al., Considering Heroin-Assisted Treatment and Supervised Drug Consumption Sites in the United States 17 (Rand Corp., Rsch. Rpt. No. 2693, 2018) (“[I]n almost all [heroin-assisted treatment] settings, participant eligibility is restricted to individuals with heroin use disorder who have tried but not responded to conventional treatments.”).
[81] See Controlled Substances Act, Pub. L. No. 91-513, 84 Stat. 1236, 1249 (1970); 21 C.F.R. § 1308.11 (2022); 21 C.F.R. § 1306.04 (2022).
[82] Strang, Groshkova & Metrebian, supra note 80, at 161. Similarly, a recent review of the literature on heroin-assisted treatment finds that “[e]vidence from all studies indicates that [heroin-assisted treatment] has benefits … for improving treatment retention and reducing illicit heroin use,” as well as in “reducing criminal activity.” Rosanna Smart, Evidence on the Effectiveness of Heroin-Assisted Treatment 43-45 (Rand Corp., Working Paper No. 1263, 2018).
[83] Kilmer et al., supra note 80, at 19.
